Electric
brain stimulation gains ground
NEUROLOGY
Electric stimulation under study to treat brain trauma from stroke to
Parkinson's and even dementia
Victoria
Colliver
Published
5:15 p.m., Tuesday, October 30, 2012
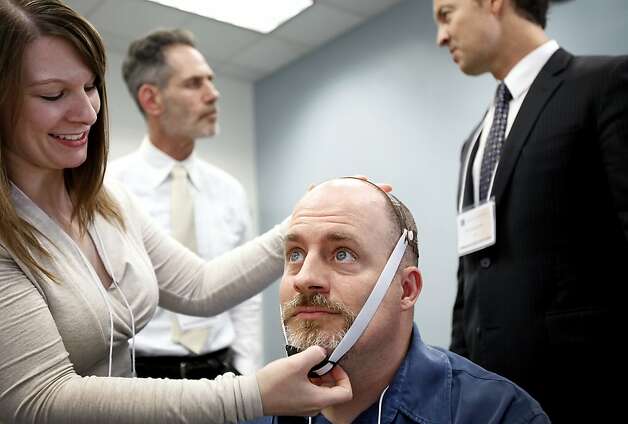
Dr. Emily Kappenman (left) prepares
psychologist Michael Callaghan for transcranial direct current stimulation. Photo:
Sarah Rice, Special To The Chronicle / SF

Dr.
Marom Bikson, who developed the transcranial direct current stimulation device,
demonstrates the product at the Highland Hospital workshop. Photo: Sarah Rice,
Special To The Chronicle / SF

Internist
Kim Wood from Joplin, Mo., watches Marom Bikson demonstrate how to use the
device. Photo: Sarah Rice, Special To The Chronicle / SF
Applying a current of electricity through the
brain conjures up the kind of nightmare-inducing seizures immortalized in the
1975 film adaption of Ken Kesey's
"One Flew Over the Cuckoo's Nest."
But a kinder, gentler, almost imperceptible
form of electric brain stimulation - an experimental approach known as
transcranial direct current stimulation - is gaining traction as a promising
therapy for brain injuries due to stroke or other traumas, depression,
dementia, attention-deficit disorder and other conditions.
Transcranial direct current stimulation, or
tDCS, bears little in common with electroshock therapy or invasive forms of
deep brain stimulation, which involve drilling holes in the head and implanting
electrodes.
The level of the current used is tiny -
typically between 1 and 2 milliamps, or less than one-one-hundredth of a single
electrical watt. At most, the current causes a tingling or slight itching, if
it's felt at all.
Even the technique's staunchest defenders
acknowledge that the idea of treating a broad range of disorders with something
you can hardly feel and that has few, if any, side effects sounds more like
snake oil than science.
"How could this do anything? It seems so
small. We're talking about a few volts," said Marom Bikson,
associate professor of biomedical engineering at City University of New York who
co-founded Soterix Medical
Inc., a company that holds patents to Bikson's transcranial direct current
stimulation devices.
Jolt to
the brain
But Bikson said studies have shown a few
volts of current can change the rate at which a brain cell fires in a way that
is believed to improve brain plasticity, or its ability to change and learn new
things.
"It's not some magical, unknown
hocus-pocus," he said.
The concept is fairly simple: After dampened
electrodes are strapped to a patient's scalp, a device charged by a 9-volt
battery - the kind used in transistor radios - delivers a small current to
change the activity in targeted regions of the brain.
The current, which is typically delivered for
10 to 30 minutes over multiple sessions, is thought to be able to excite or
inhibit the brain's neurons in the stimulated area.
A positive current could help people with
depression, stroke or other brain traumas, while a negative current may be
helpful for such conditions as epilepsy or language recovery. Both currents can
be used at the same time, and the effects of the stimulation are thought to
continue or even increase, even after the device has been turned off.
Transcranial direct current stimulation has
been used in a small number of hospitals - mostly on the East Coast - since
about 2000 but has not been approved by the U.S. Food and
Drug Administration. Soterix and other manufacturers plan to seek
FDA approval for the devices once enough research has been done.
Getting
the word out
Dr. Lance Stone, medical director of
rehabilitation and restoration at the county's Fairmont
Hospital in San Leandro, was introduced to the technique earlier
this year and invited the researchers to give his colleagues and other
specialists the opportunity to learn more about it.
Stone is interested in the device's use in
the emerging field of neurorehabilitation, which teaches or retrains patients
with nervous system injuries such as stroke, Parkinson's disease or other brain
trauma.
"There seems to be countless potential
applications (of the technique) for acquired neurological disorders, but the
main ones seem to be primarily pain, motor recovery and depression," said
Stone, who plans to apply for a research grant to study the device.
The concept of using electrical stimulation
for health purposes dates back thousands of years to the Greeks, whose medical
practitioners were said to use electric eels in water to reduce symptoms of
arthritis and other types of pain.
Modern usage of electroconvulsive therapy,
formerly known as electroshock therapy, has been controversial, dating back to
its early use in the 1940s and '50s in psychiatric hospitals. While it has been
making a bit of a lower-voltage comeback in patients with severe depression, the
method is generally considered a last resort because of the risk of memory loss
and other side effects.
Trials and
studies
Transcranial direct current stimulation has
been around for decades, but the technique earned interest in the 1990s and
early 2000s after some European physicians published promising results of the
work. Currently it's the subject of numerous ongoing clinical trials and
studies in this country.
In 2010, a team of Oxford
University scientists published a small study that showed tDCS
improved math skills in the majority of participants.
That same year, researchers at Beth Israel
Deaconess Medical Center in Boston published findings that showed
that the motor skills of stroke patients treated with the device, along with
physical and occupational therapy, improved threefold compared with those who
received a placebo form of stimulation and the same amount of physical and
occupational therapy.
But some health experts warned that the
technique's safety and effectiveness are unknown and that larger, controlled
human clinical trials are needed.
"Whenever you do something - whether
it's swallowing a drug or applying current - there may be a downside,"
said Dr. Sidney Wolfe,
director of Public Citizen's
Health Research
Group, a consumer and health advocacy lobbying organization.
Sounds
promising
Wolfe said the device sounds promising and is
the subject of a myriad of clinical trials but shouldn't be approved until the
larger, controlled studies are conducted. "The variety of medical problems
for which they are trying this is enormous, and in most of the studies the
number of patients is so small it's not statistically significant," he
said.
"The brain is an electrical organ,"
said Edwards. "What we're trying to do is develop methods that interfere
with brain activity in a targeted way and positively influence it."
Edwards said several companies in the United
States and around the world are already making transcranial direct current
stimulation devices and he expects more.
Part of the appeal of the device is that it
is relatively low cost - retailing for about $800. In addition, it's portable,
so it can be used in many different settings.
Using on
patients
Some doctors and researchers who attended the
recent workshop had no experience using the device, while others had already
tried it on a few patients. The device, which is considered investigational by
the U.S. Food and Drug
Administration, can be used either as part of a clinical trial or by
a medical doctor for specific cases.
UC Berkeley psychologist Ludovica Labruna has
experimented with using the device for language acquisition - to see if
subjects could learn languages more quickly after receiving tDCS sessions. But
she said her results have been somewhat disappointing, and she's not sure if
she's using the device correctly.
"TDCS looks so simple, but it's really
not so simple to apply because there are so many variables," Labruna said.
"You really need to be trained."
Similar concerns drew Emily Kappenman to the
workshop.
Kappenman, a postdoctoral researcher at UC
Davis' Center for the Mind and Brain, said she's interested in the device's
potential for working with patients with anxiety. She said she's tried it to
see whether it helps people become less distracted and anxious by moving their
attention away from certain emotions.
"It's hard to tell if it's working yet
or if we're using optimal levels," she said. "It's hard to know what's
best."
Brain
stimulation
Brain stimulation uses magnetic or electrical
energy to improve brain function. Here are some of the techniques in use or
being studied:
Electroconvulsive therapy (ECT): With ECT, an electric current passes briefly through the scalp to the
brain, inducing a seizure. It's generally considered only for those with severe
depression or other serious mental illnesses who do not respond to other
treatments.
Transcranial magnetic stimulation (TMS): TMS uses wrapped coil wires to generate electric current throughout the
scalp and can induce involuntary movements. Although it has a few potentially
serious side effects, including seizures, the treatment received federal
approval for patients with severe depression in 2008.
Transcranial direct current stimulation
(tDCS): Weaker still than TMS, tDCS has not been
approved, but researchers are studying its use to help patients with strokes
and other brain injuries. It has also been considered for use in healthy
subjects, with researchers testing everything from memory enhancement to
improved golf swing.
Source: Chronicle research