16 Comments
Can the Nervous System Be Hacked?
By MICHAEL BEHARMAY 23, 2014

Mirela Mustacevic, who suffers from rheumatoid arthritis, had a nerve stimulator implanted as part of a medical trial. Her symptoms have lessened significantly. Credit Sarah Wong for The New York Times
One morning in May 1998, Kevin Tracey converted a room in his lab at the Feinstein Institute for Medical Research in Manhasset, N.Y., into a makeshift operating theater and then prepped his patient — a rat — for surgery. A neurosurgeon, and also Feinstein Institute’s president, Tracey had spent more than a decade searching for a link between nerves and the immune system. His work led him to hypothesize that stimulating the vagus nerve with electricity would alleviate harmful inflammation. “The vagus nerve is behind the artery where you feel your pulse,” he told me recently, pressing his right index finger to his neck.
The vagus nerve and its branches conduct nerve impulses — called action potentials — to every major organ. But communication between nerves and the immune system was considered impossible, according to the scientific consensus in 1998. Textbooks from the era taught, he said, “that the immune system was just cells floating around. Nerves don’t float anywhere. Nerves are fixed in tissues.” It would have been “inconceivable,” he added, to propose that nerves were directly interacting with immune cells.
‘There was nothing in the scientific thinking that said electricity would do anything. It was anathema to logic. Nobody thought it would work.’
Nonetheless, Tracey was certain that an interface existed, and that his rat would prove it. After anesthetizing the animal, Tracey cut an incision in its neck, using a surgical microscope to find his way around his patient’s anatomy. With a hand-held nerve stimulator, he delivered several one-second electrical pulses to the rat’s exposed vagus nerve. He stitched the cut closed and gave the rat a bacterial toxin known to promote the production of tumor necrosis factor, or T.N.F., a protein that triggers inflammation in animals, including humans.
“We let it sleep for an hour, then took blood tests,” he said. The bacterial toxin should have triggered rampant inflammation, but instead the production of tumor necrosis factor was blocked by 75 percent. “For me, it was a life-changing moment,” Tracey said. What he had demonstrated was that the nervous system was like a computer terminal through which you could deliver commands to stop a problem, like acute inflammation, before it starts, or repair a body after it gets sick. “All the information is coming and going as electrical signals,” Tracey said. For months, he’d been arguing with his staff, whose members considered this rat project of his harebrained. “Half of them were in the hallway betting against me,” Tracey said.
Inflammatory afflictions like rheumatoid arthritis and Crohn’s disease are currently treated with drugs — painkillers, steroids and what are known as biologics, or genetically engineered proteins. But such medicines, Tracey pointed out, are often expensive, hard to administer, variable in their efficacy and sometimes accompanied by lethal side effects. His work seemed to indicate that electricity delivered to the vagus nerve in just the right intensity and at precise intervals could reproduce a drug’s therapeutic — in this case, anti-inflammatory — reaction. His subsequent research would also show that it could do so more effectively and with minimal health risks.
Tracey’s efforts have helped establish what is now the growing field of bioelectronics. He has grand hopes for it. “I think this is the industry that will replace the drug industry,” he told me. Today researchers are creating implants that can communicate directly with the nervous system in order to try to fight everything from cancer to the common cold. “Our idea would be manipulating neural input to delay the progression of cancer,” says Paul Frenette, a stem-cell researcher at the Albert Einstein College of Medicine in the Bronx who discovered a link between the nervous system and prostate tumors.
“The list of T.N.F. diseases is long,” Tracey said. “So when we created SetPoint” — the start-up he founded in 2007 with a physician and researcher at Massachusetts General Hospital in Boston — “we had to figure out what we were going to treat.” They wanted to start with an illness that could be mitigated by blocking tumor necrosis factor and for which new therapies were desperately needed. Rheumatoid arthritis satisfied both criteria. It afflicts about 1 percent of the global population, causing chronic inflammation that erodes joints and eventually makes movement excruciating. And there is no cure for it.
In September 2011, SetPoint Medical began the world’s first clinical trial to treat rheumatoid-arthritis patients with an implantable nerve stimulator based on Tracey’s discoveries. According to Ralph Zitnik, SetPoint’s chief medical officer, of the 18 patients currently enrolled in the ongoing trial, two-thirds have improved. And some of them were feeling little or no pain just weeks after receiving the implant; the swelling in their joints has disappeared. “We took Kevin’s concept that he worked on for 10 years and made it a reality for people in a real clinical trial,” he says.
Conceptually, bioelectronics is straightforward: Get the nervous system to tell the body to heal itself. But of course it’s not that simple. “What we’re trying to do here is completely novel,” says Pedro Irazoqui, a professor of biomedical engineering at Purdue University, where he’s investigating bioelectronic therapies for epilepsy. Jay Pasricha, a professor of medicine and neurosciences at Johns Hopkins University who studies how nerve signals affect obesity, diabetes and gastrointestinal-motility disorders, among other digestive diseases, says, “What we’re doing today is like the precursor to the Model T.”
The biggest challenge is interpreting the conversation between the body’s organs and its nervous system, according to Kris Famm, who runs the newly formed Bioelectronics R. & D. Unit at GlaxoSmithKline, the world’s seventh-largest pharmaceutical company. “No one has really tried to speak the electrical language of the body,” he says. Another obstacle is building small implants, some of them as tiny as a cubic millimeter, robust enough to run powerful microprocessors. Should scientists succeed and bioelectronics become widely adopted, millions of people could one day be walking around with networked computers hooked up to their nervous systems. And that prospect highlights yet another concern the nascent industry will have to confront: the possibility of malignant hacking. As Anand Raghunathan, a professor of electrical and computer engineering at Purdue, puts it, bioelectronics “gives me a remote control to someone’s body.”
Despite the uncertainties, in August, GlaxoSmithKline invested $5 million in SetPoint, and its bioelectronics R. & D. unit now has partnerships with 26 independent research groups in six countries. Glaxo has also established a $50 million fund to support the science of bioelectronics and is offering a prize of $1 million to the first team that can develop an implantable device that can, by recording and responding to an organ’s electrical signals, exert influence over its function. Instead of drugs, “the treatment is a pattern of electrical impulses,” Famm says. “The information is the treatment.” In addition to rheumatoid arthritis, Famm believes, bioelectronic medicine might someday treat hypertension, asthma, diabetes, epilepsy, infertility, obesity and cancer. “This is not a one-trick pony.”
Kevin Tracey, who is 56, came to bioelectronics because of two significant deaths. The first occurred when he was in preschool. He was 5 when his mother died as a result of an inoperable brain tumor. Shortly after the funeral, Tracey found his maternal grandfather, a professor of pediatrics at Yale, alone in his den. “I climbed onto his lap and asked what happened,” Tracey says. “He explained that surgeons tried to take it out but couldn’t separate the brain-tumor tissue from the normal neurons. I remember saying to him, ‘Somebody should do something about that.’ That was when I decided to be a neurosurgeon. I wanted to solve problems that were insolvable.”
Tracey’s second formative experience took place in May 1985. Having trained for neurosurgery at Cornell, he was on rotation for his residency in the emergency room at New York Hospital when an 11-month-old baby girl named Janice arrived in an ambulance with burns covering 75 percent of her body. Her grandmother was cooking when she tripped and doused Janice with a pot of boiling noodles. After three weeks in the burn unit recovering from skin grafts, Janice appeared to stabilize. Tracey joined Janice’s family to celebrate her first birthday in her hospital room. Janice was upbeat, smiling and giggling. The next day, she was dead.
“I was haunted by her case,” Tracey says. When the autopsy report was inconclusive, Tracey redirected his energy into medical research, specifically inflammation related to sepsis, which he believed contributed to Janice’s unexpected death. Sepsis occurs when the immune system goes into overdrive, producing a potentially lethal inflammatory response to fight a severe infection. At the time of her death, however, Janice did not have an infection. It took another year to figure out that it was an overproduction of tumor necrosis factor — the catalyst for inflammation — that caused Janice’s septic shock, though her death remains a mystery.

Kevin Tracey, a neurosurgeon, studies the effects of stimulating nerves with electricity to fight disease. Credit Katherine Wolkoff for The New York Times
“Her brakes had failed,” Tracey says. “She made too much T.N.F. The obvious question was, why?” He credits Linda Watkins, a neuroscientist at the University of Colorado, Boulder, for furnishing the pivotal clue. In the mid-1990s, Watkins was exploring possible neural connections between the brain and the immune system in rats by injecting them with cytokines — molecules that, like tumor necrosis factor, contribute to inflammation — to cause fevers. But when she cut their vagus nerves, the fever never materialized. Watkins concluded that the vagus nerve must be the conduit through which the body signals the brain to induce fever.
Tracey followed her lead by giving mice a toxin known to cause inflammation and then dosing them with an anti-inflammatory drug he had been investigating. “We injected it into their brains in teeny amounts, too small to get into their bloodstream,” he says. The drug did what it was supposed to do: It halted the production of tumor necrosis factor in the brain. Surprisingly, it also halted the production of tumor necrosis factor in the rest of the body. When Tracey cut the vagus nerve, however, the drug had no effect in the body.
“That was the eureka moment,” he says. The signal generated by the drug had to be traveling from the brain through the nerve because cutting it blocked the signal. “There could be no other explanation.”
Tracey then wondered if he could eliminate the drug altogether and use the nerve as a means of speaking directly to the immune system. “But there was nothing in the scientific thinking that said electricity would do anything. It was anathema to logic. Nobody thought it would work.”
After that first surgery on the rat in 1998, Tracey spent 11 years mapping the neural pathways of tumor-necrosis-factor inflammation, charting a route from the vagus nerve to the spleen to the bloodstream and eventually to mitochondria inside cells. “We now know more about this electrical circuit to treat [inflammation] than is known about some clinically approved drugs,” Tracey says.
By 2009, SetPoint felt ready to test Tracey’s work on people with rheumatoid arthritis, and Ralph Zitnik was approached about joining the company. “It was nuts,” Zitnik told me. “Sticking something on the vagus nerve to take away R.A.? People would think it’s witchcraft.” Zitnik’s background was in pharmaceuticals; at Amgen, he contributed to the development of Enbrel, a rheumatoid-arthritis drug that had $4.7 billion in sales last year, which made it No. 7 on the industry’s best-seller list. But the more he talked with Tracey and pored over the research, the more he said to himself: “There is good science behind this. I thought, This could work.”

SHOCK TREATMENT - VAGUS NERVE IMPLANT POD MAY 20, 2014 Illustration by Clint Ford
During a 20-minute operation, a neuVAGUS NERVE IMPLANT POD MAY 20, 2014 Illustration by Clint Fordrosurgeon will slide SetPoint Medical’s bioelectronic implant onto the vagus nerve on the left side of a patient’s neck, and then snap on an outer housing called the Pod to hold the device in place. Once the implant is activated, electrical impulses transmitted from the implant will communicate directly with immune cells in the spleen and the gastrointestinal tract, inducing them to reduce the production of cytokines — molecules that are involved in inflammation. To recharge the device’s batteries and update its software, patients and physicians will use an iPad app to control a wearable collar that transmits power and data wirelessly through the skin.
Zitnik’s first task at SetPoint was to recruit a lead scientist to set up a clinical trial. Many scientists in the United States and Europe were hesitant to do it, he says, but eventually he hired Paul-Peter Tak, a well-regarded immunologist and rheumatologist based at the Academic Medical Center, the University of Amsterdam’s teaching hospital. “He was a forward-thinking person willing to try an unconventional approach like this,” Zitnik says. Tak in turn hired Frieda Koopman, who was working on her Ph.D. in rheumatology at A.M.C., to find potential patients in the Netherlands and elsewhere in Europe.
The day after an article about the planned trial appeared in a Dutch newspaper, Koopman’s office got more than a thousand calls from rheumatoid-arthritis patients begging to participate. “We never saw that coming,” Koopman says. “We thought we might get one or two patients to join, and wouldn’t that be nice.” Invasive surgery was involved, after all. Koopman’s team returned almost every call and selected several subjects based on what medications they had tried and the severity of the pain and swelling in their joints. Over the next two years, her team continued to enroll new patients.
The subjects in the trial each underwent a 45-minute operation. A neurosurgeon fixed an inchlong device shaped like a corkscrew to the vagus nerve on the left side of the neck, and then embedded just below the collarbone a silver-dollar-size “pulse generator” that contained a battery and microprocessor programmed to discharge mild shocks from two electrodes. A thin wire made of a platinum alloy connected the two components beneath the skin. Once the implant was turned on, its preprogrammed charge — about one milliamp; a small LED consumes 10 times more electricity — zapped the vagus nerve in 60-second bursts, up to four times a day. Typically, a patient’s throat felt constricted and tingly for a moment. After a week or two, arthritic pain began to subside. Swollen joints shrank, and blood tests that checked for inflammatory markers usually showed striking declines.
Koopman told me about a 38-year-old trial patient named Mirela Mustacevic whose rheumatoid arthritis was diagnosed when she was 22, and who had since tried nine different medications, including two she had to self-inject. Some of them helped but had nasty side effects, like nausea and skin rashes. Before getting the SetPoint implant in April 2013, she could barely grasp a pencil; now she’s riding her bicycle to the Dutch coast, a near-20-mile round trip from her home. Mustacevic told me: “After the implant, I started to do things I hadn’t done in years — like taking long walks or just putting clothes on in the morning without help. I was ecstatic. When they told me about the surgery, I was a bit worried, because what if something went wrong? I had to think about whether it was worth it. But it was worth it. I got my life back.”
In February, I met Moncef Slaoui, Glaxo’s chairman of Global Research and Development, at one of the company’s 16 facilities he oversees worldwide, this one in King of Prussia, Pa. Slaoui, who is 55 and has a Ph.D. in molecular biology and immunology, was instrumental in developing the first malaria vaccine and is considered one of the most influential executives in the pharmaceutical industry.
“When Kris came to me in early 2012 with this idea of vagus nerve stimulation,” Slaoui told me, “I was like: C’mon? You’re gonna give a shock and it changes the immune system? I was very skeptical. But finally I agreed to visit Kevin’s lab. I wanted the data, the evidence. I don’t like hot air.” He went to Tak, the lead scientist for the trials. “I asked him, ‘Paul-Peter, is it really real?’ ”

SetPoint Medical’s new neural implant (currently being tested on animals). Credit Katherine Wolkoff for The New York Times
After getting an endorsement from Tak, who is now Glaxo’s global head of immuno-inflammation research, Slaoui committed to financing SetPoint. The investment was modest, though, because he felt that Tracey’s device was “just a starting point. It was still very broad — you touch the vagus nerve, you touch most of your viscera. We had wanted something very specific.” What he didn’t want was “the bulldozer approach” that characterizes already existing stimulators for treating Parkinson’s, chronic pain and epilepsy. (Pacemakers differ because they stimulate muscle, not nerves.) These devices are indiscriminate, blasting electricity into billions of neurons and hoping for the best. As Slaoui saw it, SetPoint’s stimulator was a primitive forerunner to “a device that reads your electrical impulses and sees when something is wrong, then corrects what needs correcting.”
In 2006, Slaoui continued, “when I became chairman of R. & D., R. & D. was a liability to this company. We were spending lots of money and not producing new molecules for new medicines. I had to acknowledge that the current way of doing R. & D. wasn’t likely to be successful.” Four years later, Slaoui put together a 14-member think tank and discussed, among other topics, the Human Brain Project. The multinational endeavor, directed by the neuroscientist and Fulbright scholar Henry Markram, at the Swiss Federal Institute of Technology in Lausanne, is trying to create a computer simulation of the human brain. That got Slaoui “thinking about electrical signaling, an opportunity to make medicine — a therapeutic intervention — that’s super highly specific in terms of its geographic position. I’m going to go to the nerve that goes to your kidney and nowhere else, and only to your left kidney, and to a particular area of the left kidney.”
That degree of precision would address one of Slaoui’s major criticisms of conventional drugs: They flood the body, and then doctors have to hope that they will perform only where they’re supposed to. “It is really difficult to design a molecule that will only interact where you want it, because it goes everywhere.” The upshot, usually: side effects. Bioelectronics could potentially eliminate those, as well as the costly redundancy involved in the drug-discovery process, in which every promising molecule must be independently evaluated. “There is very little that is transposable from one molecule to the next,” Slaoui said. “You have to redo everything.” Bioelectronics attracted him, he says, because “95 percent of the hardware is the same,” no matter what disease it treats.
So Slaoui found himself working for a drug company while devoting himself to the idea of treating illness without drugs. In July 2012, he and Famm toured Markram’s facilities in Lausanne. There Markram showed them a 3-D digital visualization on a giant screen of 100,000 synapses actively firing in a mouse brain.
At that moment, Famm says, he and Slaoui realized they were “biting off too much.” Slaoui and Famm concluded that starting with the brain — which seemed logical, given that it’s the body’s C.P.U. — could take decades to yield viable treatments. The human brain’s circuitry, with 100 billion neurons, seemed far too complex. “Why don’t we just skip the brain and go straight to the organs?” Slaoui suggested.
Right then, Slaoui said, “we decided to focus on the peripheral nervous system.” The peripheral nerves link the brain and spinal cord (the central nervous system) to the organs and limbs. Rather than try to fathom the brain — a black box, basically, with its 100 trillion neural connections — Slaoui proposed that they put “an interface between a nerve and the organ with an electrical device.” To eavesdrop on a telephone call, his thinking went, you don’t tap into the switching center and search for the conversation. You go to the line nearest the caller’s location. Compared with the brain, the cablelike bundles that are the peripheral nerves contain vastly fewer fibers — hundreds versus billions.
The brain, with its billions of neurons, seemed far too complex. ‘Why don't we just skip the brain and go straight to the organs?’ someone suggested.
When I joined Famm in Philadelphia in February, he referred to his role as Glaxo’s bioelectronics chief as “like being a missionary.” Famm, who lives in London, was in the U.S. to attend half a dozen meetings with bioelectronics researchers. His challenge is coaxing those from disparate disciplines to embrace a singular vision. Whereas drug discovery primarily involves like-minded thinkers — molecular biologists, chemists, geneticists — bioelectronics calls for alliances between experts in fields that in many cases have little to do with medicine — nanotech, optics, electrical engineering, materials science, computer programming, wireless networking and data mining. At the moment, Famm is focused on getting what he called a “transdisciplinary” group of scientists to agree on how to solve two key technical challenges.
The first is shrinking the hardware. It must be small enough to attach to virtually any nerve yet still have enough battery power and circuitry to run algorithms that generate the patterns of electrical impulses needed to treat various diseases. At the Charles Stark Draper Laboratory in Cambridge, Mass., we met with a team working on miniaturization. Draper is best known for internal navigation systems that guide things like ballistic missiles and spaceships. Bryan McLaughlin, who directs bioelectronics development at Draper, showed me the latest prototype mock-up — a dime-size implant. It’s small, he said, but not nearly small enough. McLaughlin wants to get its electrodes, microprocessor, battery and a wireless transmitter into a device no larger than a jelly bean. “It’s also important to make it closed-loop, with the ability to read and write to the nervous system.” The goal, in other words, is to end up with something that can continuously monitor a patient and then dispense bioelectronic therapy as needed.
The second challenge is devising a method to make sense of signals emanating simultaneously from hundreds of thousands of neurons. Accurate recording and analysis are essential to bioelectronics in order for researchers to identify the discrepancies between baseline neural signals in healthy individuals and those produced by someone with a particular disease. The conventional approach to recording neural signals is to use tiny probes with electrodes inside called patch clamps. A prostate-cancer researcher, for example, could attach patch clamps to a nerve linked to the prostate in a healthy mouse and record the activity. The same thing would be done with a mouse whose prostate had been genetically engineered to produce malignant tumors. Comparing the output from both might allow the researcher to determine how the neural signals differ in cancerous mice. From such data, a corrective signal could be programmed into a bioelectronic device to treat the cancer.
using patch clamps. They can sample only one cell’s activity at a time, and therefore fail to gather enough data to see the big picture. As Adam E. Cohen, who teaches chemistry and physics at Harvard, puts it, “It’s like trying to watch an opera through a straw.”
Cohen, an expert in an emerging field called optogenetics, thinks he can overcome the limitations of the patch clamps. His research is trying to use optogenetics to decipher the neural language of disease. “Getting patch clamps into a single [neuron] is extremely slow and laborious — about an hour per cell,” Cohen told me when I visited his lab recently. “The bigger problem is that [neural] activity comes not from the voices of individual neurons but from a whole orchestra of them acting in relation to each other. Poking at one at a time doesn’t give you the global view.”
Optogenetics arose out of a series of developments in the 1990s. Scientists knew that proteins, called opsins, in bacteria and algae generated electricity when exposed to light. Optogenetics exploits this mechanism. Opsin genes are inserted into the DNA of a harmless virus, which is then injected into the brain or a peripheral nerve of a test subject. By choosing a virus that prefers some cell types over others, or by altering the virus’s genetic sequence, researchers can target specific neurons — cold- or pain-sensing, for example — or regions of the brain known to be responsible for certain actions or behaviors. Next, an optical fiber — a spaghetti-thin glass cable that transmits light from its tip — is inserted through the skin or skull to the site of the virus. The fiber’s light activates the opsin, which in turn conducts an electrical charge that forces the neuron to fire. Researchers have already controlled mouse behavior with optogenetics — inducing sleep and aggression on command.
Instead of drugs, says the man who runs GlaxoSmithKline's bioelectronics research and development, ‘the treatment is a pattern of electrical impulses. The information is the treatment.’
Before opsins can be used to activate neurons involved in specific ailments, however, scientists must determine not only which neurons are responsible for a particular disease but also how that disease communicates with the nervous system. Like computers, neurons speak a binary language, with a vocabulary based on whether their signal is on or off. The specific sequence, interval and intensity of these on-off shifts determine how information is conveyed. But if each disease can be thought of as speaking its own language, then a translator is needed. What Cohen and others recognized was that optogenetics can do that job. So Cohen reverse-engineered the process: Instead of using light to activate neurons, he used light to record their activity.
Cohen showed me his “Optopatch” machine. It consisted of red and blue lasers, mirrors, lenses, a high-speed digital camera, a video projector, a microscope and several quiet cooling fans. After he turned it on, a postdoc fellow who works in his lab, Shan Lou, inserted a petri dish under its microscope. The dish contained 11 live neural cells from mice, harvested from dorsal-root ganglia, which relay sensory input to the brain. Lou added a few drops of capsaicin extract, the irritant in pepper spray, and then turned the camera on for 14 seconds. In that brief period, it snapped 7,000 frames, totaling 12 gigabytes of data. To analyze it, Cohen had written software that searches for patterns by employing techniques developed for digital voice and face recognition. “We also use algorithms and optical tricks derived from astrophysics,” Cohen said. Seconds later, an analysis appeared on Lou’s computer screen. Three of the 11 cells had been identified as firing in response to the capsaicin, indicating that they were pain-sensing neurons. It would have taken Cohen more than a day to record and make sense of that cellular information with a patch clamp. This sort of effort was a step, he said, “toward imaging large numbers of neurons in parallel, hundreds, perhaps thousands.”
Cohen is collaborating with Ed Boyden, a professor of neuroscience at M.I.T. and a pioneer in optogenetics, to develop the so-called closed-loop implant envisioned by Bryan McLaughlin at Draper Labs. Optogenetics, Boyden told me, enables him to “aim light at some subset of cells [without] activating all the stray cells nearby.”
Opsins might point the way to future treatments for all kinds of diseases, but researchers will most likely have to develop bioelectronic devices that don’t use them. Using genetically engineered viruses is going to be tough to get past the F.D.A. The opsin technique hinges on gene therapy, which has had limited success in clinical trials, is very expensive and seems to come with grave health risks.
Cohen mentions two alternatives. One involves molecules that behave like opsins; another uses RNA that converts into an opsinlike protein — because it doesn’t alter DNA, it doesn’t have the risks associated with gene therapy. Neither approach is very far along, however. And “you still face the problem of getting the light in,” he says. Boyden is developing a brain implant with a built-in laser, but Cohen believes an external light source is more likely for most bioelectronics applications.
Surmounting these sorts of technical hurdles “might take 10 years,” Famm figures. That seems somewhat optimistic if you consider Glaxo’s investment so far in bioelectronics. Melinda Stubbee, the company’s director of communications, says it has spent roughly $60 million in the area, a pittance compared with its $6.5 billion in total R. & D. expenditures in 2013. Slaoui, defending the number, said, “Funding of R. & D. is like an investment” — money only flows toward bankable ideas. While he thinks the area shows promise, he seems to want independent researchers to do the legwork before Glaxo buys in further.
‘I think this is the industry that will replace the drug industry,’ says a pioneer in bioelectronics.
At one point, Famm referred to detractors who say bioelectronics is “too risky, will take too long and is maybe even a bit bonkers.” In trying to find some of them, I contacted a number of financial analysts who track Glaxo and the pharmaceutical industry. One, Mark Clark, at Deutsche Bank, said to me in an email: “I know next to nothing about this early-stage technology! I am prepared to bet you will not find a single Glaxo analyst that knows anything about this! Research technologies were a vogue thing to be expert on in the ’90s and tech-bubble years, but we only care about drugs that are actually in the clinical pipeline these days, not how they get there — to be brutally blunt!”
In short, the fledgling bioelectronics industry is nowhere near mature enough for analysts to make meaningful estimates about its revenue potential. But people like Clark will certainly begin paying closer attention if bioelectronics starts to capture even a sliver of the lucrative pharmaceutical market. Drug sales for rheumatoid arthritis alone were $12.3 billion in 2012. That looks like a big opportunity to an outfit like SetPoint.
Yet if large numbers of patients someday choose bioelectronics over drugs, another issue awaits resolution: security. Bioelectronics devices will feature wireless connectivity so they can be fine-tuned and upgraded, “just like the software on your iPhone,” Famm says. And wireless means hackable, an unsettling fact that worries two experts on medical-device security: Niraj Jha, a professor of electrical engineering at Princeton University, and Anand Raghunathan, who runs the Integrated Systems Laboratory at Purdue.
Fears of medical devices being hacked aren’t new. In 2007, Dick Cheney’s cardiologist disabled the wireless functionality in the former vice president’s defibrillator to prevent terrorists from trying to stop his heart. Jha and Raghunathan, along with the lead author, Chunxiao Li, detailed how this might be accomplished in a seven-page paper they wrote, “Hijacking an Insulin Pump,” published in June 2011. The paper described a hack they performed in their lab using inexpensive, off-the-shelf hardware.
According to Jha and Raghunathan, there are no known cases of malicious attacks on medical devices. Nevertheless, Raghunathan says, “Society should be warned about these possibilities.” The Department of Homeland Security is no doubt worried, addressing the potential threat in an alert it issued last June. In August, the F.D.A. offered guidelines to medical-device manufacturers, recommending “wireless protection” to reduce “risks to patients from a security breach.” Whether bioelectronics developers do anything to thwart hacking (the F.D.A. guidelines are not mandatory) may ultimately depend on whether Jha and Raghunathan’s fears are realized.
Draper’s McLaughlin doesn’t dismiss these concerns but notes that there is no “incentive for device companies to do anything about security.” He adds: “Nobody has been sued. No patient has died. But the first event that occurs with one of these devices — companies will jump on it and create secure platforms.”
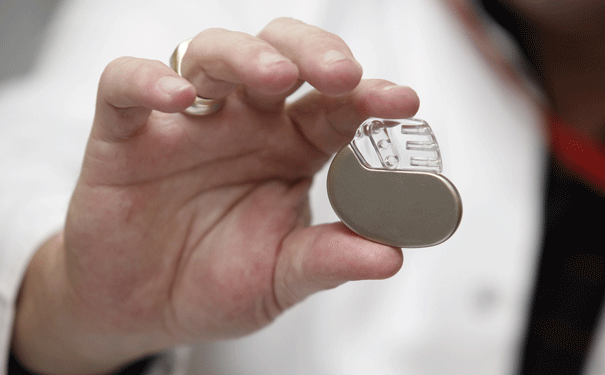
SetPoint’s chief technology officer is Mike Faltys, a medical engineer who was integral to designing the modern cochlear implant. Faltys worked for six years out of his garage, first re-engineering an existing electrical stimulator, used to stop seizures, that became the device implanted in patients in SetPoint’s trial, and more recently finishing a significantly more advanced implantable unit that he calls “the microregulator.”
Housed in a pod shaped like a hot-dog bun and the size of a multivitamin, the entire microregulator is entirely self-contained — onboard battery, microprocessor and electrodes are integrated into a single unit. It can be wirelessly recharged, and adjusted and updated with an iPad app. The surgery to clamp it onto the vagus nerve will take about 20 minutes, and once in place, it will provide pain relief to a rheumatoid-arthritis patient for a decade or more before it needs servicing.
On one occasion during my travels with Famm, I got to hold SetPoint’s newfangled microregulator. For now, it’s only capable of transmitting very crude signals to communicate with the nervous system — more like grunts and groans rather than the precise vocabulary that Slaoui envisions for bioelectronic therapies. Even so, the microregulator felt elegant and powerful and promising in my palm. “A patient gets a device like this implanted once for one disease, and they’re done,” Tracey says. “No prescriptions, no medicines, no injections. That’s the future. That’s what gets me out of bed in the morning.”
Michael Behar writes about science and the environment. His work has appeared in “The Best American Travel Writing” and “The Best American Science and Nature Writing.”
http://www.nytimes.com/2014/05/25/magazine/can-the-nervous-system-be-hacked.html?emc=eta1&_r=0
16 Comments
![]() Explore further: Deep brain stimulation for obsessive-compulsive disorder releases dopamine in the brain
Explore further: Deep brain stimulation for obsessive-compulsive disorder releases dopamine in the brain ![]()
![]()