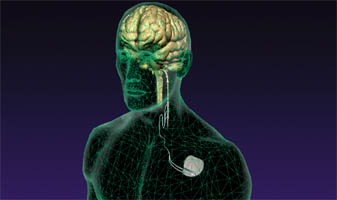
Cyberonics Announces 100,000th Patient Implant of VNS Therapy®
Announcement highlights important patient milestone during 25th anniversary year
HOUSTON, Dec. 20, 2012 /PRNewswire/ -- Cyberonics, Inc. (NASDAQ: CYBX) today announced the 100,000th patient implant of its Vagus Nerve Stimulation (VNS) Therapy system. The announcement comes as Cyberonics recognizes a milestone year marking the 25th anniversary of the company's founding. The VNS Therapy System for the treatment of epilepsy received CE Mark approval 18 years ago, and this year marks the 15th anniversary of approval by the United States Food and Drug Administration (FDA).
According to an Institute of Medicine Report released earlier this year, approximately one in 26 people will develop epilepsy at some point during their lives. VNS Therapy is a unique device-based treatment option specifically developed for people with refractory epilepsy. Epilepsy is considered refractory when seizures are not adequately controlled by medications alone or when the side effects associated with seizure medications are intolerable. Recently-published data from New York University clinician researchers show that more than 60 percent of people with refractory epilepsy treated with adjunctive VNS Therapy experience 50 percent or greater reduction in seizures. Clinician researchers from Emory University have also shown in a recent publication that adding VNS Therapy to the treatment regimen of patients experiencing refractory epilepsy is associated with significant reductions in resource utilization (ER visits, hospitalizations, and inpatient days) and epilepsy‐related clinical events, as well as a net cost savings after 1.5 years.
"Cyberonics is proud to acknowledge this important milestone and the impact VNS Therapy has made on the lives of so many people with epilepsy and their families," said Dan Moore , Cyberonics' President and Chief Executive Officer. "We look forward to expanding access to VNS Therapy and innovating new therapeutic options for patients around the world."
VNS Therapy device technology has evolved over time to become smaller, have a longer battery life and offer new programming functionalities. The latest model of VNS Therapy, the AspireHC® generator, is available in the U.S. and in Europe. The AspireHC generator offers physicians and patients additional options, such as longer battery life, improved electronics and enhanced programming features. Cyberonics continues to develop new device-based solutions for epilepsy, including the ProGuardian™ system, an innovative, in-home monitoring system; the AspireSR™ generator, a VNS Therapy system featuring automatic magnet-mode stimulation; and Relay™, a wireless-enabled generator.
http://www.prnewswire.com/news-releases/cyberonics-announces-100000th-patient-implant-of-vns-therapy-184314661.html